ClinOleic
An olive oil-based lipid emulsion that is associated with fewer infections than soybean oil and may preserve immune function.1-4

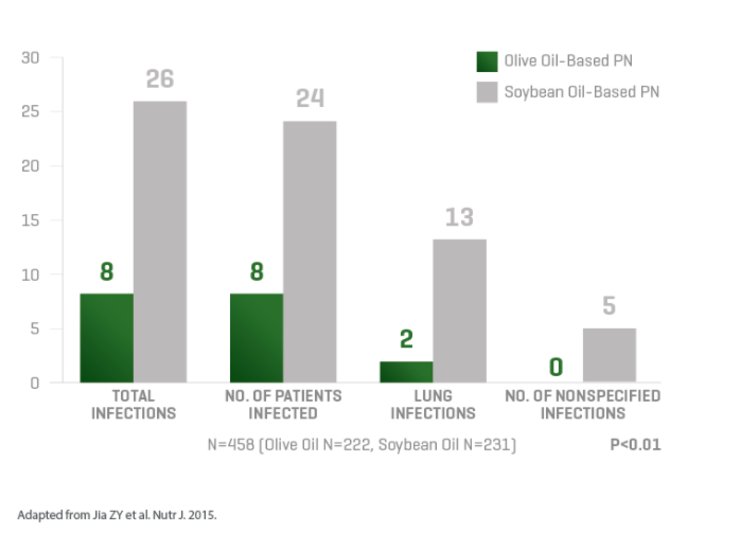
Lipids are an integral part of parenteral nutrition (PN), but some lipid emulsions, such as 100% soybean oil, may modulate patient immune response and increase the risk of complications.3,5 Through 20 years of experience, ClinOleic maintains the lowest soybean oil content of any soy-containing composite lipid emulsion.6-11 ClinOleic also contains 80% olive oil, which has been associated with fewer infections and may preserve immune function.1-4

Aligned with international guidelines
ASPEN guidelines recommend to withhold or limit soybean oil-based lipid emulsions during the first week following the initiation of PN.18 Additionally, ESPEN guidelines recommend that lipids based solely on soybean oil be avoided.14 ClinOleic has the lowest soybean oil content of any soy-containing composite lipid product on the market, enabling the delivery of essential fatty acids while minimizing the unwanted effects of soybean oil.6-11,15

ClinOleic
ClinOleic is the lipid emulsion in Baxter’s three-chamber bags Olimel and Numeta. Download the Baxter Parenteral Nutrition Compatibility Guide for compounding compatibility and stability information.
Learn more about Clinical Nutrition

Related Products
Important safety information
This abbreviated summary of product characteristics (SPC) is intended for international use. Please note that it may differ from the licensed SPC in the country where you are practicing.
Therefore, please always consult your country-specific SPC or package leaflet.
ClinOleic 20%:
Emulsion for infusion.
QUALITATIVE AND QUANTITATIVE COMPOSITION
Per 100 ml: Refined olive oil and refined soybean oil* 20.00 g corresponding to a content of essential fatty acids 4.00 g. Energy content 2000 kcal/l (8.36 MJ/l).Lipid content (olive and soybean oil) 200 g/l.Osmolarity 270 mOsm/l.pH 6 -8 Density 0.986.Phospholipids provide 47 milligrams or 1.5 mmol of phosphorus per 100 ml.
*Mixture of refined olive oil (approximately 80%) and refined soybean oil (approximately 20%).
CLINICAL PARTICULARS
Therapeutic indications:
Indicated as a source of lipids for patients requiring parenteral nutrition, when oral or enteral nutrition is impossible, insufficient or contraindicated.
Posology and method of administration:
ClinOleic 20% contains 200 mg/ml of lipids.
The posology depends on energy expenditure, the patient’s clinical status, body weight, and ability to metabolize ClinOleic 20%, as well as additional energy given orally/enterally. Therefore, the dosage should be individualized and the bag size chosen accordingly.
Dosage:
In adults: The posology is 1 to a maximum of 2 g lipids/kg/day. The initial infusion rate must be slow and not exceed 0.1 g lipids or 0.5 ml (10 drops) per minute for 10 minutes then gradually increased until reaching the required rate after half an hour. Never exceed 0.15 g lipids/kg/hour (0.75 ml/kg/hour). Adults per kg of body weight: Usual lipid dosage: 1 to 2 g/kg/day. Infused volume of ClinOleic 20%:5 to 10 ml/kg/day. Adults for 70 kg: Usual lipid dosage: 70 to 140 g/day. Infused volume of ClinOleic 20%: 350 to 700 ml/day.
In children: ClinOleic 20% should be administered as a continuous 24h/day infusion. It is recommended not to exceed a daily dose of 3g-lipids/kg b.w. and an infusion rate of 0.15 g lipids/kg b.w./h. Daily dose should be increased gradually during the first week of administration.
In premature newborns and low birth weight infants: The use of ClinOleic 20% is restricted to premature infants of 28 weeks of gestational age or more. ClinOleic 20% should be administered as a continuous 24h/day infusion. The initial daily dose should be 0.5-1.0g lipids/kg b.w. The dose may be increased by 0.5-1.0g lipids/kg b.w. every 24 hours up to a daily dose of 2.0 g lipids/kg b.w. Usage in nutritive admixtures (with glucose and amino acids): “Breaking” or “oiling out” of the emulsion can be visibly identified by accumulation of yellowish droplets or particles in the admixture.
Contraindications:
- hypersensitivity to the active substance or to any of the excipients (e.g.: egg or soybean protein)
- severe dyslipidemia and non-corrected metabolism disorders including lactic acidosis and uncompensated diabetes
Special warnings and special precautions for use:
The infusion must be stopped immediately if any abnormal signs or symptoms of an allergic reaction (such as sweating, fever, shivering, headache, skin rashes or dyspnoea) develop. This medicinal product contains soybean oil and egg phospholipids. Soybean and egg proteins may cause hypersensitivity reactions. Cross-allergic reactions between soybean and peanut proteins have been observed.
During short-term or long-term intravenous nutrition, alkaline phosphatases and total bilirubin should be checked at regular intervals, depending on the health status of the patient. The blood sugar, serum triglycerides, the acid-base balance, electrolytes, serum osmolarity, kidney function, coagulation parameters and the blood count must be checked at regular intervals.
Parenteral nutrition should be used with caution in patients with pre-existing liver disease or liver insufficiency. Liver function parameters should be closely monitored in these patients.
Parenteral Associated Liver Diseases (PNALD) including cholestasis, hepatic staetosis, fibrosis and cirrhosis, possibly leading to hepatic failure, as well as cholecystitis and cholelithiasis are known to develop in some patients on parenteral nutrition. The etiology of these disorders is thought to be multifactorial and may differ between patients. Patients developing abnormal laboratory parameters or other signs of hepatobiliary disorders should be assessed early by a clinician knowledgeable in liver diseases in order to identify possible causative and contributory factors, and possible therapeutic and prophylactic interventions.
ClinOleic 20% should be administered with caution in case of neonatal hyperbilirubinemia (total serum bilirubin > 200 μmol/ l). Total bilirubin levels should be monitored closely. As other lipid emulsions, ClinOleic 20% should be used in extremely premature and/or very low birth-weight infant under the close supervision of a neonatologist. There is clinical experience for ClinOleic 20% infusion time, up to 7 days in neonates and up to 2 months in children. Light exposure of solutions for intravenous parenteral nutrition, especially after admixture with trace elements and/or vitamins, may have adverse effects on clinical outcome in neonates, due to generation of peroxides and other degradation products. When used in neonates and children below 2 years, Clinoleic should be protected from ambient light until administration is completed
Interactions with other medicaments and other forms of interaction: Complete information about incompatibilities is not available. ClinOleic 20% contains vitamin K, naturally present in lipid emulsions. The amount of Vitamin K in recommended doses of ClinOleic 20%, is not expected to influence effects of coumarin derivatives.
The lipids contained in this emulsion may interfere with the results of certain laboratory tests if the blood sample is taken before the lipids are eliminated from the serum (these are generally eliminated after a period of 5 to 6 hours without receiving lipids). Refer to the laboratory testing system product information regarding potential assay interference associated with lipemic samples.
Pregnancy and lactation: The safety of administration of ClinOleic 20% during pregnancy and lactation has not been established. Therefore, ClinOleic 20% should not be used during pregnancy and lactation except after special consideration.
Undesirable effects:
Common: nausea vomiting, hyperglycaemia, mean arterial pressure decreased.
Uncommon: Dyspnea, Cholestasis, Blood bilirubin increased, Hepatic enzyme increased.
Overdose: A reduced ability to remove the lipids may result in a “fat overload syndrome” which may be caused by overdose, the effects of which are usually reversible after the lipid infusion is stopped.
PHARMACEUTICAL PARTICULARS:
List of excipients:
Egg phospholipids, glycerol, sodium oleate, sodium hydroxide, water for injections.
Incompatibilities:
Complete information about incompatibilities is not available. Never add medication or electrolytes directly to the lipid emulsion. If it is necessary to introduce additives, verify the compatibility and mix thoroughly before administration to the patient.
Shelf life:
18 months in plastic bag in its overwrap. When used in neonates and children below 2 years, the solution (in bags and administration sets) should be protected from light exposure until administration is completed.
Special precautions for storage:
Do not store above 25°C.Do not freeze. Keep the container in the outer carton.
Medicinal products subject to medical prescription.
Date of preparation: Mar 2020